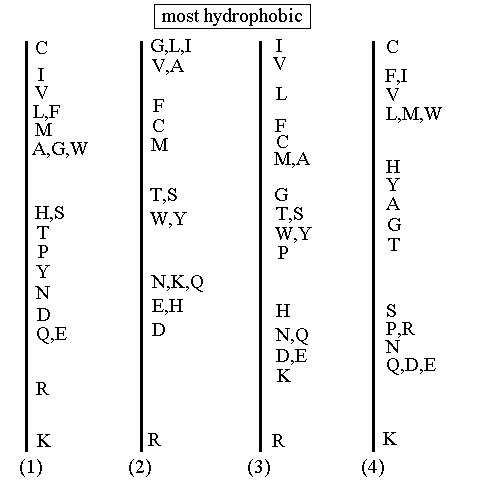

Figure 1. A diagram comparing four different scales for the "hydrophobicity" of an amino acid residue in a protein [4]. References for the scales are as follows: (1) Janin (1979)1; (2) Wolfenden, et al.2; (3) Kyte and Doolittle 3; and (4) Rose, et al.4.
It is clear that there are significant differences between the four scales shown above, with respect to the placement of specific amino acids. 1 & 4 both place cysteine as the most hydrophobic residue, while the other two scales do not. The reason for this difference is the fundamentally different methods used for constructing the scales. Scales 1 & 4 were both constructed by examining proteins with known 3-D structures and defining hydrophobic character as the tendency for a residue to be found inside of a protein, rather than on its surface. In the case of cysteine, because it is involved in disulphide bonds that must necessarily occur inside of a globular structure, cysteine appears to be extremely hydrophobic.
Scales 2 & 3 are derived from the physicochemical properties of amino acid side chains and therefore more clearly follow the trends that would be expected, on the inspection of amino acid structures.
An excellent review of the various "hydrophobicity" scales (37 in all) that exist is given by Cornette, et al.5. This paper evaluates the various scales and attempts to extract correlations from the various types of data presented by the scales. It is a very mathematical paper, but the second Appendix is a valuable reference for the various types of scales available in the literature.
Charton & Charton6 offer some insight into why so many scales have developed. In their conclusion of a theoretical study of "hydrophobicity", they state:
"Hydrophobicity parameters and log P values show a variable dependence
on intermolecular forces and steric effects. It seems very likely that
no single hydrophobicity parameter or log P value can represent the
complete range of amino acid behavior.
There is no special phenomenon denoted by hydrophobicity in amino
acids. It is the natural and predictable result of differences in the
intermolecular forces between water and the amino acid and those between
the amino acid and some other medicum. No "hydrophobic bonds" need to be
invoked to account for amino acid behavior."
| 1. | J. Janin, Surface and Inside Volumes in Globular Proteins,Nature, 277(1979)491-492. |
| 2. | R. Wolfenden, L. Andersson, P. Cullis and C. Southgate,Affinities of Amino Acid Side Chains for Solvent Water,Biochemistry 20(1981)849-855. |
| 3. | J. Kyte and R. Doolite, A Simple Method for Displaying the Hydropathic Character of a Protein, J. Mol Biol. 157(1982)105-132. |
| 4. | G. Rose, A. Geselowitz, G. Lesser, R. Lee and M. Zehfus,Hydrophobicity of Amino Acid Residues in Globular Proteins, Science 229(1985)834-838. |
| 5. | J. Cornette, K. B. Cease, H. Margalit, J. L. Spouge, J. A. Berzofsky and C. DeLisi, Hydrophobicity Scales and Computational Techniques for Detecting Amphipathic Structures in Proteins, J. Mol. Biol. 195(1987)659-685. |
| 6. | M. Charton and B. I. Charton, The Structural Dependence of Amino Acid Hydrophobicity Parameters, J. theor. Biol. 99(1982)629-644. |